Abstract
Background
The outcome of DLBCL in the elderly is well described in clinical trials with reported 2-year OS of 60-80%. However less well reported are aspects of care of elderly DLBCL patients treated outside the trial context, including rates of completion of induction chemotherapy and detailed reasons for dose delays and reductions and their effect on outcomes including remission rates and progression free survival (PFS). Here we describe an Australian experience of management of elderly DLBCL patients treated at 3 large institutions.
Methods
This is a multi-center, retrospective analysis of consecutive patients aged >=60, with DLBCL, planned to receive rituximab-chemotherapy . The standard regimens included 3 cycles (early stage disease) or 6 cycles (advanced) RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) followed by two rituximab doses in fit patients; R-miniCHOP for unfit and R-CE(etoposide)OP for patients with cardiacdysfunction. Disease response was PET-based. Patient data including included baseline characteristics, histology, clinical parameters, comorbidities, treatment outcomes and toxicity were extracted from hospital medical records. The primary endpoint was PFS; secondary endpoints included overall survival (OS), overall response rate and toxicity and rates of dose delays, dose reductions, hospital admission and completion of planned treatment. Descriptive statistics were used in the analysis.
Results
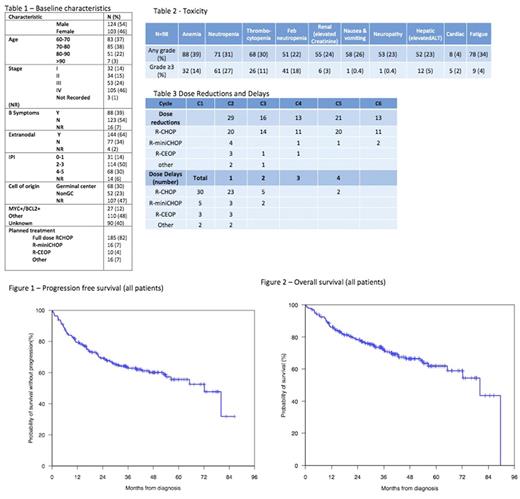
227 patients were evaluated from 2012-2017. Table 1 shows baseline characteristics and treatment. Median age was 73 years (range 60-97). Major comorbidities were present in 126 (55%) patients, with ischaemic heart disease (24%), diabetes (21%) and renal impairment (15%) the most common. Impaired cardiac function (EF<55%) was present in 25 (11%) of cases. G-CSF was used in 57 (25%) patients for primary prophylaxis and 20 (9%) patients post febrile neutropenia. Dose reductions occurred in 114 patients (50%): 59 (26%) upfront, 85 (38%) from cycle 2 onwards and 30 (13%) requiring both. Fifty-four patients (24%) had more than one dose reduction during treatment and 40 (18%) had dose delays. Only 19 (8%) patients did not receive all planned cycles of R-chemotherapy. Hospital admission was required in 167 (74%) cases. The median number of admissions was 4 (range 0-16) with a median inpatient duration of 7 days (range 1-83). Sixteen patients were admitted to ICU and 36 had limitations place to their resuscitation orders due to admission related morbidity. Of the evaluable patients (one center) for toxicity (n=98) the most common (12, 5%) grade 3-4 non-haematological toxicity was hepatic (ALT>2xULN), see table 2. Complete remission (CR) on PET following completion of treatment was achieved in 161 cases (71%) and was not impacted by dose reduction/delay (CR of 71% in these cases). Radiotherapy was administered in 56 (25%) patients, predominantly for initial bulky disease (48%) or for residual disease (40%). Median follow-up was 33 months. Two year PFS 69% was (95% CI 63-76%), OS 78% (95% CI 73-84%), see figures 1 and 2 for survival curves. The impact of dose reduction, dose delay and major co-morbidities on survival are under assessment and will be presented at the meeting.
Discussion
In our cohort, DLBCL in the elderly presented frequently as advanced stage, with high rates of B symptoms and extranodal disease. Clinically-relevant comorbidities were present in the majority of patients. Despite >90% of patients completing the planned number of treatment cycles, toxicity was significant with a high rate of hospital admission and dose reductions. In this real world setting, CR rates and survival were reassuringly comparable with published trial data. Elderly DLBCL patients with significant comorbidities have similar survival to the published data but close monitoring and early intervention for toxicity is needed.
Hawkes: Roche: Other: Travel expenses; Bristol-Myers Squibb: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Other: Travel expenses, Research Funding; Merck Sharpe Dohme: Research Funding; Takeda: Other: Travel expenses; Celgene: Research Funding; Merck Serono: Research Funding. Grigg: Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses. Cheah: BMS: Membership on an entity's Board of Directors or advisory committees, Other: Conference travel expenses; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Research Funding, Speakers Bureau; Celgene: Research Funding; Abbvie: Research Funding; Amgen: Other: Conference travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal